

| Program name | ปรัชญาดุษฎีบัณฑิต (เภสัชศาสตร์และเทคโนโลยี) Doctor of Philosophy (Pharmaceutical Sciences and Technology) |
|||||||||||||||||||||||||||||||||||||||||||||
| Banner picture | ||||||||||||||||||||||||||||||||||||||||||||||
| Degree awarded |
Fullname(Thai) หลักสูตรปรัชญาดุษฎีบัณฑิต สาขาวิชาเภสัชศาสตร์และเทคโนโลยี (หลักสูตรนานาชาติ) (หลักสูตรปรับปรุง พ.ศ. 2566) Fullname(English) Doctor of Philosophy Program in Pharmaceutical Sciences and Technology (International Program)(Revised 2023) Initials name(Thai) ปร.ด. (เภสัชศาสตร์และเทคโนโลยี) Initials name(English) Ph.D. (Pharmaceutical Sciences and Technology) |
|||||||||||||||||||||||||||||||||||||||||||||
| Awarding body & teaching institution | Faculty of Pharmacy, Silpakorn University, Sanam Chandra Palace Campus, Nakhon Pathom | |||||||||||||||||||||||||||||||||||||||||||||
| The program specification was written on | The revised program 2023 will be effective from the first semester of the academic year 2023
The curriculum was approved by Faculty of Pharmacy Committee at the meetings 6/2022 on 7 April 2022 The curriculum was approved by Academic Committee at the meetings 7/2022 on 6 July 2022 The curriculum was approved by Silpakorn University Academic Council at the meetings 9/2022 on 26 September 2022 The curriculum was approved by Silpakorn University Council at the meetings 10/2022 on 19 October 2022 The curriculum was approved by Ministry of Higher Education, Science, Research and Innovation on 10 July 2023 |
|||||||||||||||||||||||||||||||||||||||||||||
| Program learning outcomes (PLOs) | 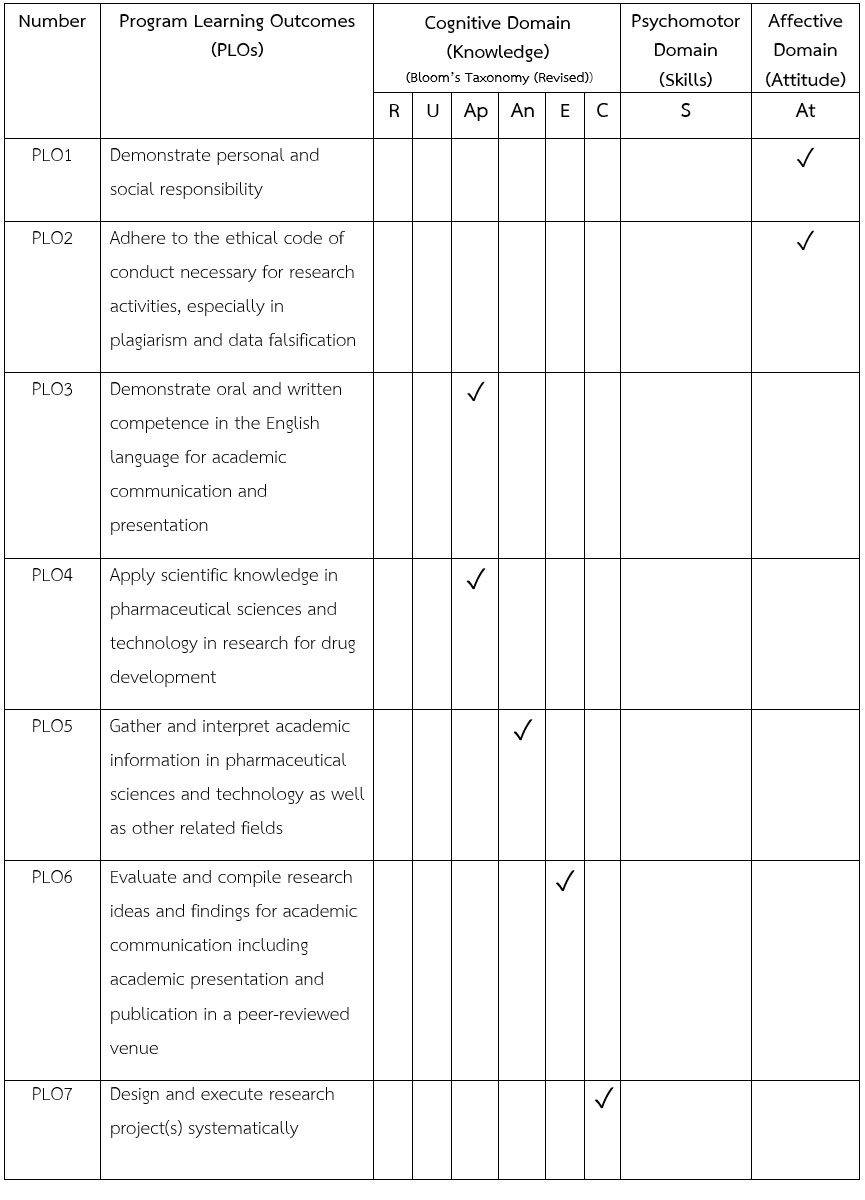 |
|||||||||||||||||||||||||||||||||||||||||||||
| Benchmark related to PLOs | ||||||||||||||||||||||||||||||||||||||||||||||
| Study plan | Type 1 Thesis only 1) Type 1.1: Number of total credits for the program 48 credits 2) Type 1.2: Number of total credits for the program 72 credits Type 2 Thesis and additional course works 1) Type 2.1: Students are required to earn a minimum of 48 credits 2) Type 2.2: Students are required to earn a minimum of 72 credits |
|||||||||||||||||||||||||||||||||||||||||||||
| Admission criteria | Entry Requirement
1) Requirements of a study plan as the followings: 1.1) Graduates of a Bachelor’s degree in pharmacy, health sciences or sciences and technology or related fields with a “very good” level of GPA or equivalent or 1.2) Graduates of a Master’s degree in pharmacy, or other related fields of study with a “good” level of GPA or equivalent 2) English examination result according to the committee of Higher Education council or the announcement of Silpakorn University as of standard English proficiency test for the admission of doctorate study 3) Accordance with Silpakorn University’s 2018 Regulations on Graduate Study and/or later revision (see Appendix A) and/or updated amendment. Other qualifications may also apply as reviewed by the Committee of the Faculty of Pharmacy. |
|||||||||||||||||||||||||||||||||||||||||||||
| Program structure |
Type 1.1
Type 1.2
Type 2.1
Type 2.2
|
|||||||||||||||||||||||||||||||||||||||||||||
| Career path options | 1) Researcher in pharmaceutical sciences and technology
2) Academic in pharmaceutical sciences and technology 3) Consultant in pharmaceutical sciences and technology for government and private sectors 4) Lecturer of pharmaceutical sciences and technology for graduate study in a public and private university 5) Self-employed business |
Year 1 / First Semester
| Course Code | Subject | Number of Credits (L – P – S) |
| 574 701 | Seminar in Pharmaceutical Sciences and Technology I | 1*(0-3-0) |
| Total | 0 | |
Year 1 / Second Semester
| Course Code | Subject | Number of Credits (L – P – S) |
| 574 702 | Seminar in Pharmaceutical Sciences and Technology II | 1*(0-3-0) |
| Total | 0 | |
Year 2 / First Semester
| Course Code | Subject | Number of Credits (L – P – S) |
| 574 752 | Thesis (equivalent to) | 12 |
| Total | 12 | |
Year 2 / Second Semester
| Course Code | Subject | Number of Credits (L – P – S) |
| 574 752 | Thesis (equivalent to) | 12 |
| Total | 12 | |
Year 4 / First Semester
| Course Code | Subject | Number of Credits (L – P – S) |
| 574 752 | Thesis (equivalent to) | 12 |
| Total | 12 | |
Year 3 / Second Semester
| Course Code | Subject | Number of Credits (L – P – S) |
| 574 752 | Thesis (equivalent to) | 12 |
| Total | 12 | |
Year 1 / First Semester
| Course Code | Subject | Number of Credits (L – P – S) |
| 574 701 | Seminar in Pharmaceutical Sciences and Technology I | 1*(0-3-0) |
| 574 703 | Research Methodology in Pharmaceutical Sciences and Technology | 3*(3-0-6) |
| 574 705 | Theoretical Aspects of Pharmaceutical Sciences and Technology | 3*(3-0-6) |
| Total | 0 | |
Year 1 / Second Semester
| Course Code | Subject | Number of Credits (L – P – S) |
| 574 702 | Seminar in Pharmaceutical Sciences and Technology II | 1*(0-3-0) |
| 574 704 | Equipment in Pharmaceutical Sciences and Technology | 3* (2-3-4) |
| Total | 0 | |
Year 2 / First Semester
| Course Code | Subject | Number of Credits (L – P – S) |
| 574 753 | Thesis (equivalent to) | 12 |
| Total | 12 | |
Year 2 / Second Semester
| Course Code | Subject | Number of Credits (L – P – S) |
| 574 753 | Thesis (equivalent to) | 12 |
| Total | 12 | |
Year 3 / First Semester
| Course Code | Subject | Number of Credits (L – P – S) |
| 574 753 | Thesis (equivalent to) | 12 |
| Total | 12 | |
Year 3 / Second Semester
| Course Code | Subject | Number of Credits (L – P – S) |
| 574 753 | Thesis (equivalent to) | 12 |
| Total | 12 | |
Year 3 / First Semester
| Course Code | Subject | Number of Credits (L – P – S) |
| 574 753 | Thesis (equivalent to) | 12 |
| Total | 12 | |
Year 4 / Second Semester
| Course Code | Subject | Number of Credits (L – P – S) |
| 574 753 | Thesis (equivalent to) | 12 |
| Total | 12 | |
Year 1 / First Semester
| Course Code | Subject | Number of Credits (L – P – S) |
| 574 701 | Seminar in Pharmaceutical Sciences and Technology I | 1(0-3-0) |
| Elective Courses | 3 | |
| Total | 4 | |
Year 1 / Second Semester
| Course Code | Subject | Number of Credits (L – P – S) |
| 574 702 | Seminar in Pharmaceutical Sciences and Technology II | 1(0-3-0) |
| Elective Courses | 3 | |
| Total | 4 | |
Year 2 / First Semester
| Course Code | Subject | Number of Credits (L – P – S) |
| Elective Courses | 4 | |
| Total | 4 | |
Year 2 / Second Semester
| Course Code | Subject | Number of Credits (L – P – S) |
| 574 751 | Thesis (equivalent to) | 12 |
| Total | 12 | |
Year 3 / First Semester
| Course Code | Subject | Number of Credits (L – P – S) |
| 574 751 | Thesis (equivalent to) | 12 |
| Total | 12 | |
Year 3 / Second Semester
| Course Code | Subject | Number of Credits (L – P – S) |
| 574 751 | Thesis (equivalent to) | 12 |
| Total | 12 | |
Year 1 / First Semester
| Course Code | Subject | Number of Credits (L – P – S) |
| 574 701 | Seminar in Pharmaceutical Sciences and Technology I | 1(0-3-0) |
| 574 703 | Research Methodology in Pharmaceutical Sciences and Technology | 3(3-0-6) |
| 574 705 | Theoretical Aspects of Pharmaceutical Sciences and Technology | 3(3-0-6) |
| Elective Courses | 3 | |
| Total | 10 | |
Year 1 / Second Semester
| Course Code | Subject | Number of Credits (L – P – S) |
| 574 702 | Seminar in Pharmaceutical Sciences and Technology II | 1(0-3-0) |
| 574 704 | Equipment in Pharmaceutical Sciences and Technology | 3(2-3-4) |
| Elective Courses | 3 | |
| Total | 7 | |
Year 2 / First Semester
| Course Code | Subject | Number of Credits (L – P – S) |
| Elective Courses | 7 | |
| Total | 7 | |
Year 2 / Second Semester
| Course Code | Subject | Number of Credits (L – P – S) |
| 574 752 | Thesis (equivalent to) | 8 |
| Total | 8 | |
Year 3 / First Semester
| Course Code | Subject | Number of Credits (L – P – S) |
| 574 752 | Thesis (equivalent to) | 10 |
| Total | 10 | |
Year 3 / Second Semester
| Course Code | Subject | Number of Credits (L – P – S) |
| 574 752 | Thesis (equivalent to) | 10 |
| Total | 10 | |
Year 4 / First Semester
| Course Code | Subject | Number of Credits (L – P – S) |
| 574 752 | Thesis (equivalent to) | 10 |
| Total | 10 | |
Year 4 / Second Semester
| Course Code | Subject | Number of Credits (L – P – S) |
| 574 752 | Thesis (equivalent to) | 10 |
| Total | 10 | |
Remark * refers to non-credit courses
Type 1.1 and Type 2.1 Curriculum
Regular period of study = 3 years
Type 1.2 and Type 2.2 Curriculum
Regular period of study = 4 years
| 574 701 | Seminar in Pharmaceutical Sciences and Technology I
Condition: Type 1.1 and 1.2 graded as S/U, Type 2.1 and 2.2 graded as per the 4.00 rating scale Searching, retrieving and compiling scientific data of research topics in pharmaceutical sciences and technology from various sources focusing on overview or general knowledge obtained from the selected topic. Analysis of collected information for rational discussion and presentation. |
1(0-3-0) |
| 574 702 | Seminar in Pharmaceutical Sciences and Technology II
Condition: Type 1.1 and 1.2 graded as S/U, Type 2.1 and 2.2 graded as per the 4.00 rating scale Searching, retrieving and compiling scientific data of research topics in pharmaceutical sciences and technology from various sources focusing on the in-depth knowledge and experimental design. Analysis of collected information for rational discussion and presentation. |
1(0-3-0) |
| 574 703 | Research Methodology in Pharmaceutical Sciences and Technology
Condition: Type 1.1 and 1.2 graded as S/U, Type 2.1 and 2.2 graded as per the 4.00 rating scale A systematic approach in conducting a research study including the selection of research topic, planning, and design of a research project. Operational concepts of research. Populations. Samples. Parameters and data. Research proposal preparation. Data collection. Statistics for research. Analysis and interpretation of research data. Research work dissemination. Research ethics in pharmaceutical sciences and technology. |
3(3-0-6) |
| 574 704 | Equipment in Pharmaceutical Sciences and Technology
Condition: Type 1.1 and 1.2 graded as S/U, Type 2.1 and 2.2 graded as per the 4.00 rating scale Theories, principles, techniques, and practices of commonly encountered unit operations in pharmaceutical sciences and technology and related areas. |
3(2-3-4) |
| 574 705 | Theoretical Aspects of Pharmaceutical Sciences and Technology
Condition: Type 1.1 and 1.2 graded as S/U, Type 2.1 and 2.2 graded as per the 4.00 rating scale Theories and principles in pharmaceutical sciences and technology. Dosage form design and evaluations. Research and development of pharmaceutical products. |
3(3-0-6) |
| 574 711 | Current Topics in Pharmaceutical Sciences and Technology
Novel concepts based on current information and trend in pharmaceutical sciences and technology. Research and development of new drugs and delivery systems with required specifications. |
2(2-0-4) |
| 574 712 | Special Problem in Pharmaceutical Sciences and Technology
Selected topics in pharmaceutical sciences and technology and related fields. Data collection and analysis including research skills to solve problems. |
1(0-3-0) |
| 574 713 | Progress in Cosmetic Sciences and Technology
Theories, principles, advanced skills in novel cosmetic sciences and technology for design formulation. Evaluation and manufacturing of cosmetic products emphasizing medicated cosmetics. Skin properties that enhance or limit the efficacy of medicated cosmetics. Future trends in cosmetic development. |
3(2-3-4) |
| 574 714 | Advances in Drug Delivery Technology
Concepts and principles of advanced drugs and biomolecules delivery technology. Focusing on the current trends of high potential delivery systems, including macroscale to particulate technology for therapeutic and diagnostic applications. |
3(3-0-6) |
| 574 715 | Principles of Pharmaceutical Engineering
Requirements for the design of facilities, equipment, and processes in the pharmaceutical and related industries including facility layout and principles of design. Planning and construction of critical facilities emphasizing the water system, ventilation system, and environmental system. Manufacturing process validation. |
3(3-0-6) |
| 574 716 | Advanced Organic Pharmaceutical Chemistry
Principles and theories of advanced organic pharmaceutical chemistry for explaining mechanisms of drug action and drug stability. Strategies for solving problems in drug discovery and development. |
3(3-0-6) |
| 574 717 | Spectroscopic Techniques in Pharmaceutical Sciences
Structure identification of organic compounds based on spectroscopic data, including applications in drug discovery, drug development, and quality control of drug substances as well as pharmaceutical products. |
3(3-0-6) |
| 574 718 | Drug Design and Synthesis
Theories and principles of drug design. Drug synthesis and related chemical reaction mechanisms. Drug purification and structure identification. |
3(2-3-4) |
| 574 719 | Advanced Instrumental Methods of Analysis
Theories and analytical techniques using advanced instruments involving spectroscopic, chromatographic, and chemometric methods for qualitative and quantitative analysis of pharmaceutical substances and impurities. |
4(2-6-4) |
| 574 720 | Special Problems in Advanced Pharmaceutical Chemistry
Selected topics in advanced pharmaceutical chemistry emphasizing the advancement and techniques in pharmaceutical chemistry and analysis. Experimentation and interpretation of research results. |
3(1-6-2) |
| 574 721 | Advanced Cellular and Molecular Biology
In-depth understanding of the molecular biology of the cells emphasizes on structure and functions of biomolecules, cellular organization, cell motility, cell communication, signal transduction, cell regulation, and pathogenesis-related to dysregulation of the cell. |
3(3-0-6) |
| 574 722 | Advanced Biotechnology in Pharmaceutical Sciences
In-depth study in theories, methods, techniques, and applications of biotechnology for pharmaceutical research and development. |
3(2-3-4) |
| 574 723 | Bioinformatics in Pharmaceutical Sciences
Application of bioinformatics tools to compute and/or analyze biopharmaceutical data. Data retrieving. Sequence and structural analysis of genetic materials and protein, including design of experimental bioinformatics models. |
2(1-3-2) |
| 574 724 | Cellular and Molecular Microbiology
Cellular and molecular biology of microorganisms. Host-pathogen interaction. Pathogen-antimicrobial agent interaction. Molecular technologies for diagnosis. Epidemiology. Antimicrobial discovery. |
3(2-3-4) |
| 574 725 | Cellular and Molecular Pharmacology
Pharmacological principles and mechanism of action of drugs at an organ, tissue, cellular and molecular level. Integrating pharmacological principles and mechanisms of drug action at the organ, tissue, cellular and molecular levels for drug discovery and development. |
3(3-0-6) |
| 574 726 | Pharmacology and Toxicology in Nonclinical Drug Development
Principles of pharmacological and toxicological studies involved in non-clinical drug discovery and development. |
3(3-0-6) |
| 574 727 | Pharmacogenomics
Principles of pharmacogenomics in drug response. Definition of precision medicine and its applications. The impact of pharmacogenetics on the use of drugs and the pharmaceutical industry. |
3(3-0-6) |
| 574 728 | Research Principle in Pharmacology and Toxicology
Principles and practice of research studies in pharmacology and toxicology. |
3(1-6-2) |
| 574 729 | Separation Techniques of Natural Products
Theory and principle in the extraction and isolation of natural products. Application of separation and bioactivity-guided fractionation techniques to obtain the potential extracts/ compounds from natural products. |
3(2-3-4) |
| 574 730 | Structure Elucidation of Natural Compounds
Principle and methodology of structure elucidation techniques of natural compounds. Determination of the chemical structure of compounds isolated from natural products. |
3(2-3-4) |
| 574 731 | Analysis of Natural Products
Theory and principle of techniques in natural product analysis; method validation of analyzing processes. Application of the techniques for the analysis of natural products. |
3(2-3-4) |
| 574 732 | Phytochemistry
Plant chemistry. Sources of plant compound. Chemical properties of plant compound. Bioactivities of phytochemicals. |
3(3-0-6) |
| 574 751 | Thesis
Conducting a research study on pharmaceutical sciences and technology under the supervision of thesis advisors. |
equivalent to 36 credits |
| 574 752 | Thesis
Conducting a research study on pharmaceutical sciences and technology under the supervision of thesis advisors. |
equivalent to 48 credits |
| 574 753 | Thesis
Conducting a research study on pharmaceutical sciences and technology under the supervision of thesis advisors. |
equivalent to 72 credits |

